
APHRODITE-155 PRIN PNRR 2022 (2024-2025)

APHRODITE-155 is the acronym of “Accelerator-based Production of tHeranostic radionuclides: Investigations on TErbium-155” funded as PRIN PNRR 2022 for the years 2024-2025 (“Finanziato dall’Unione europea – Next Generation EU”). The production of the theranostic radionuclide 155Tb, currently not available on the international market, has gained the attention of the researchers working in the field of radiopharmaceuticals as Auger-and Conversion Electrons emitter with a suitable γ-ray for SPECT (Single Photon Emission Computed Tomography) imaging, as already underlined by the REMIX project. 155Tb can also be coupled to other Tb-isotopes, composing emerging theranostic pairs and innovative tools for fighting cancer with personalized treatments in nuclear medicine. The study of 155Tb therapeutic properties can also shed much light on the true cytotoxic power of Auger-and Conversion Electrons emitters for targeted radionuclide therapy, since 155Tb does not emit additional alpha or beta particles, as shown in the Figure. However, the production of significant amounts of 155Tb is still an open issue.
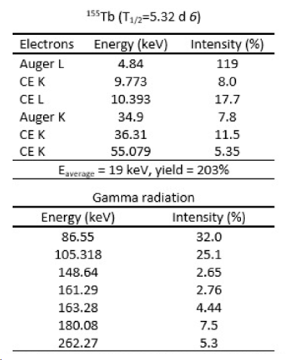
Main decay characteristics of 155Tb
Recently, it was shown that among the low-energy routes only the 155Gd(p,n) reaction can provide 155Tb with a suitable radionuclidic purity (RNP) for medical applications. On the other hand, the 159Tb(p,5n)155Dy reaction, feasible at intermediate-energy proton cyclotron as the SPES one installed at the INFN-LNL, provides 155Dy, the precursor of 155Tb, and can be suitable for a generator-system supply. APHRODITE-155 aims to assess the best 155Tb production route by using cyclotrons, comparing low and intermediate routes, considering the Thick Target Yield (TTY), isotopic purity (IP) and RNP of the final 155Tb. Both experimental and theoretical data will be used to assess the optimal 155Tb production, also considering the dose increase due to the presence of eventual contaminant radionuclides for specific 155Tb-labelled radiopharmaceuticals. In-house 155Tb production at the Sacro Cuore Don Calabria Hospital (SCDCH, Negrar, VR) and at INFN-LNL will be performed, including thick target manufacture, irradiation runs, optimization of a suitable radiochemical process and quality controls (QC). For the low-energy route, isotopically-enriched [155Gd]Gd2O3 thick targets and the optimisation of a suitable radiochemical process for Tb/Gd will be developed; this radiochemical procedure needs to be automatised to guarantee maximum reproducibility and radiation-protection of the operator. For the intermediate-energy 159Tb(p,5n)155Dy reaction, the monoisotopic thick targets will be irradiated at INFN-LNL and transported to UNIMI unit for Dy/Tb radiochemical separation and subsequently elute the 155Tb produced from electron capture decay of 155Dy. In both cases, γ-spectrometry measurements are essential to accurately evaluate 155Tb quality to properly define its future use in preclinical and clinical studies. To test the feasibility of 155Tb-labelled radiopharmaceuticals, dosimetric analysis will be also carried out, paving the way for further in vitro and in vivo tests. The fundamental expertise of the INFN (PI of the project and responsible of the INFN unit: G. Pupillo), UNIFE (responsible of the UNIFE unit: P. Martini) and UNIMI (responsible of the UNIMI unit: F. M. Groppi Garlandini) units, in collaboration with the SCDCH, the Istituto Oncologico Veneto (IOV, PD), and the Physics Departments of Padova and Pavia Universities are the ground of this project (Collaborations).